Subretinal Gene Therapy Drug laru-zova (AGTC-501) for X-Linked Retinitis Pigmentosa (XLRP) Phase 1/2 Multicenter Study (Horizon): 24-Month Interim Safety Results

ROBERT E. MACLAREN, DPHIL, FMEDSCI, FRCOPHTH, FRCS, FACS UNIVERSITY OF OXFORD, UK
Disclosures
- REM is a director and co-founder of Beacon Therapeutics
- REM is a consultant to AGTC and Beacon Therapeutics
- REM is a named inventor on a patent for RPGR gene therapy
laru-zova (AGTC-501) (AAV.coRPGR)
- One-time therapy to target the genetic cause of XLRP
- Replaces mutated RPGR genes using a proven vector
- Designed to create a full-length protein
- Subretinal delivery has demonstrated:
- Safety in animals and humans
- Clinical efficacy in animals and humans
- Further being developed by the ongoing clinical development program
ORF15 Region of RPGR Gene
The RPGR protein has two major isoforms
- RPGR1-19: widely expressed in tissues and the mature photoreceptor connecting cilia
- RPGRORF15: mostly found in the retina and photoreceptor connecting cilia
The ORF15 region of the RPGR gene includes a glutamic acid and glycine-rich region
- This region has been challenging to stabilize for the production of gene therapy vectors
- Glutamylation is significantly reduced with large in-frame deletions within the ORF15 region
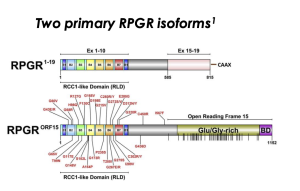
RPGRORF15 consists of exons 1-14 of RPGR1-19 and ORF15, a combination of exon 15 and intron 15
Potential Therapeutic Benefits of Using Full-length RPGRORF15
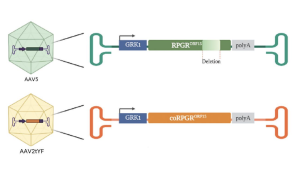
- Beacon uses a stable, full-length RPGRORF15 gene therapy vector, overcoming the pitfalls of a truncated RPGRORF15
- As a full-length RPGR gene therapy, laru-zova (AGTC-501) therefore has a higher probability of restoring the natural function of cone photoreceptors, yielding greater visual improvement
- laru-zova (AGTC-501) and BIIB112 (Biogen) express the same correct full-length RPGR protein and undergo full glutamylation during posttranslational modification.
Focus on Cone Function
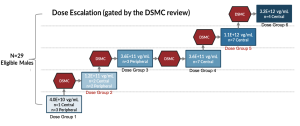
Phase 1/2 Horizon Study Design
An Open-Label, Dose Escalation Study to Evaluate the Safety and Efficacy of laru-zova (AGTC-501) in Patients with XLRP Caused by RPGR Mutations
Primary Endpoint
Safety
Key Secondary Endpoints
Change from baseline in:
- Visual function in centrally treated (MAIA)
- BCVA (ETDRS)
- Retinal structure (SD-OCT)
- FPI: 16 April 2018 • 5-year follow-up post treatment
Key Takeaways: Horizon Month 24 Interim Analysis
- Safety
- Favorable safety profile over 24 months
- No suspected unexpected serious adverse reactions (SUSARs), no endophthalmitis
- No SAEs deemed related to study agent
- Efficacy
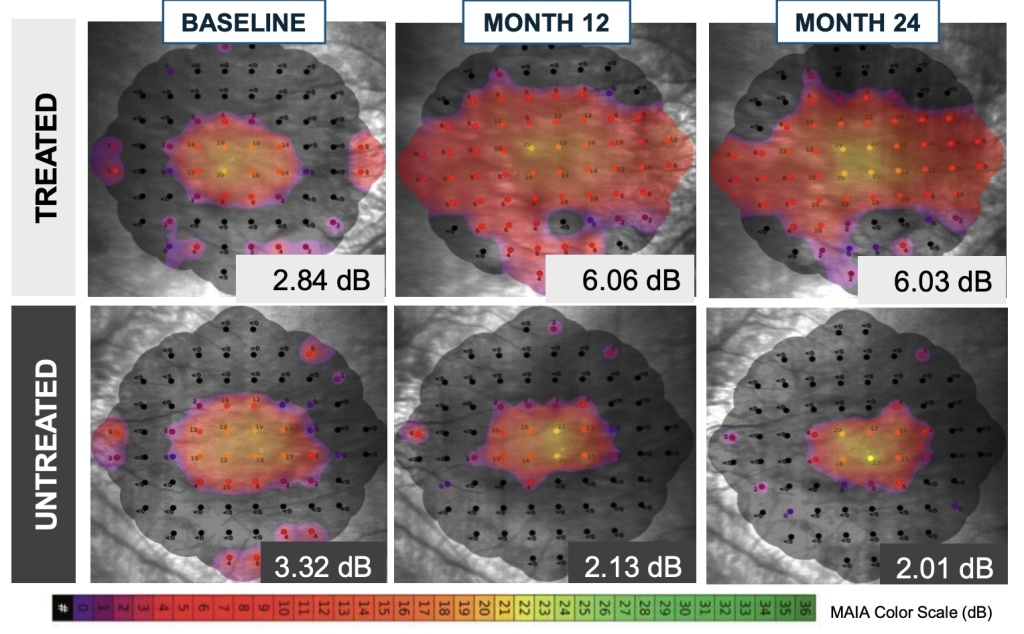
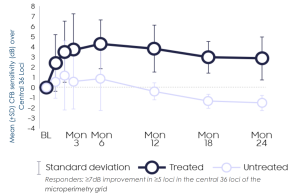
- Compared with the fellow untreated eyes, significant treatment effect in visual sensitivity across central 36 loci and loci within bleb was seen and sustained thru Month 24
- BCVA continues to show supportive evidence of a biological response at Month 24
- Improvements in retinal sensitivity continued to correlate with improvements in retinal structure
Horizon: Improvement in retinal sensitivity at 3 months, persisting out to 24 months
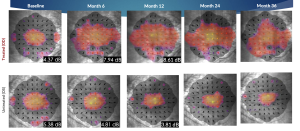
Microperimetry Mapping in a Responder Eye
Conclusions: laru-zova (AGTC-501) Phase 1/2 Horizon XLRP 24-Month Interim Safety Results
No clinically significant safety events related to the study agent
- Generally safe and well tolerated over 24 months
- No significant vision loss
- No cone dysfunction
- The overall safety profile is consistent with a subretinal procedure, vitrectomy procedure, and concomitant corticosteroid treatment
- Continued clinical development strongly supported
- Encouraging efficacy outcomes
- laru-zova (AGTC-501) expresses the full-length RPGR protein in photoreceptor cells
Next Steps: Continue Clinical Development
Plan to initiate two clinical studies by first quarter 2024
- DAWN (US)
- Open-label, fellow-eye dosing study
- Will enroll fellow (untreated) eye of participants previously dosed with an AAV vector-based gene therapy designed to provide a full-length functioning RPGR protein
- VISTA (EU and US)
- Phase 2/3, randomized, controlled, masked, multi-center study
- Evaluating 2 doses of laru-zova (AGTC-501) and an untreated control group
Thank you!
WE WOULD LIKE TO THANK THE INVESTIGATORS, SITES, AND STUDY PARTICIPANTS AND THEIR FAMILIES WHO PARTICIPATED IN THIS STUDY





